Second Law Of Thermodynamics
- Heat will never be seen to flow spontaneously from cooler object to hotter object.
- The entropy of an isolated system never decreases it either remain constant (reversible process) or increases (reversible process).
- The total entropy of any system and that of the surroundings always increases.
Entropy:
Is defined as the number of ways a system can be arranged. The higher the entropy
(meaning the more ways the system can be arranged), the more the system is disordered. Another example of this definition of entropy is illustrated by spraying perfume in the corner of a room. We all know what happens next. The perfume will not just stay in that corner of the room. The perfume molecules will eventually fill up the room. The perfume went from an ordered state to a state of disorder by spreading throughout the room and that is one of the example of entropy.
What is the spontaneous process and not spontaneous process?
One of the main uses of 2nd Law of Thermodynamics process is to determine weather process is a spontaneous or not.
And that is an example of spontaneously process or not spontaneous process.
Change in entropy:
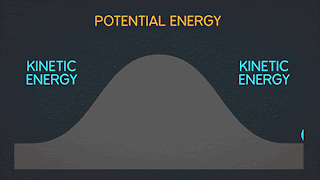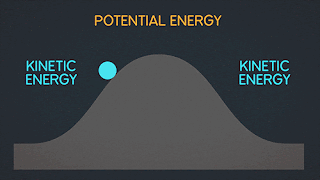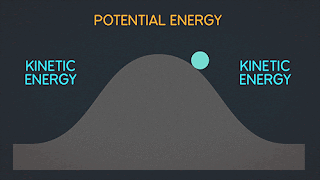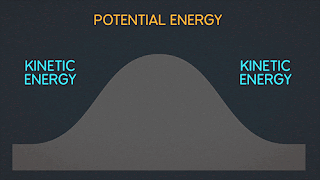
Lets take look in this process when change of entropy occur and have an irreversibly process.
As you can see in the picture above the gas are stock in left side of a sealed container because of our insulating wall. but when our insulating wall break or open. The gas rushes to fill right volume and This is an irreversible process; all the molecules of the gas will never return to the left half of the container.
(meaning the more ways the system can be arranged), the more the system is disordered. Another example of this definition of entropy is illustrated by spraying perfume in the corner of a room. We all know what happens next. The perfume will not just stay in that corner of the room. The perfume molecules will eventually fill up the room. The perfume went from an ordered state to a state of disorder by spreading throughout the room and that is one of the example of entropy.
As we can see two system have an arrange system but when they collide each other the system disarrange in any order and that is another example of entropy.
What is the spontaneous process and not spontaneous process?
One of the main uses of 2nd Law of Thermodynamics process is to determine weather process is a spontaneous or not.
Example of spontaneous process and not spontaneous process
for example, ice will spontaneously melt, but water will not spontaneously freeze.
and in order to freeze the water we comes work effort to make the water freeze.
Change in entropy:
Lets take look in this process when change of entropy occur and have an irreversibly process.
NOTE:
For a reversible process change in entropy is.
where Q is the heat transfer, which is postive for heat transfer into negative for heat transfer out of, and T is the absolute temperature at which the reversible process takes place. the SI unit for entropy is joules per kelvin (J/K) if temperature changes during the process, then it is usually a good approximation ( for small changes in temperature) to take T to be the average temperature, avoiding the need to use integeral calculus to find 
this is an example video of this topic:
zeroth law:
The Zeroth Law of Thermodynamics states that if two bodies are each in thermal equilibrium with some third body, then they are also in equilibrium with each other. Thermal equilibrium means that when two bodies are brought into contact with each other and separated by a barrier that is permeable to heat, there will be no transfer of heat from one to the other
I'll give another idea of zeroth law:
the zeroth law of thermodynamics which states that " if body "A" and body are in thermal equilibrium and body "B" and body "C" are in the thermal equilibrium and then body "A" and "C" are will also in thermal equilibrium. see this picture above to understand very well.
KEY POINTS:
- Assuming A, B, and C are three systems, if A and C are in thermal equilibrium, and A and B are in thermal equilibrium, then B and C are in thermal equilibrium.
- Two systems are in thermal equilibrium if they could transfer heat between each other, but do not.
- The Zeroth Law of Thermodynamics implies that temperature is a quantity worth measuring.
UNDERSTANDING EQUILIBRIUM:
Most generally, equilibrium refers to a balanced state that does not change overall with time. This does not mean that nothing is happening; rather, that two influences or forces are balancing each other out.
Consider, for example, a weight hanging from a string attached to the ceiling. At first, the two are in equilibrium with one another and the string does not break. If more weight is attached to the string, however, the string will be tugged downward and may eventually break as the two are no longer in equilibrium.
SHORT QUIZ:
1. three substances are added to a mug to make coffee : the coffee which is 65 °C the milk which is 65 ° C and the sugar which is in thermal equilibrium with the
coffee describe the thermal state of sugar.
coffee describe the thermal state of sugar.
Answer :
The sugar is in equilibrium with the milk, based on the zeroth law of thermodynamics
2.System A is in equilibrium with system C. System B is in equilibrium with system C.System A is in equilibrium with system B according to which law of thermodynamics?
Answer:
The zeroth law of thermodynamics
3. Gas A is in thermal equilibrium with gases B and C. Which of the following is a valid conclusion?
Thermal equilibrium of gas B is indirectly proportional to that of gas C
Gases B and C are in thermal equilibrium with each other
Thermal equilibrium of gas B is directly proportional to that of gas C
Answer :
Gases B and C are in thermal equilibrium with each other
4.If I know that the change in entropy of a system where heat was added is 20 J/K, and that the temperature of the system is 300 K, what is the amount of heat added to the system?
Answer:
6000 J.
5.If you add 400 J of heat to a system so that the final temperature of the system is 200 K, what is the change in entropy of the system?
Answer:
2 J/K.
6.If you know that the change in entropy of a system where heat was added is 40 J/K, and that the temperature of the system is 200 K, what is the amount of heat added to the system?
Answer:
8000 J.
7.If you know that the change in entropy of a system where heat was added is 12 J/K, and that the temperature of the system is 250 K, what is the amount of heat added to the system?
Answer:
3000 J.
8.If you know that the change in entropy of a cup of coffee where heat was added is 20 J/K, and that the temperature of the coffee is 250 K, what is the amount of heat added to the cup of coffee?
Answer:
5000 J.
9.If you add 10 J of heat to a system so that the final temperature of the system is 200K, what is the change in entropy of the system?
Answer:
0.05 J/K.
10.If you know that the change in entropy of a system where heat was added is 40 J/K, and that the temperature of the system is 200 K, what is the amount of heat added to the system?
Answer :
8000 J
references:
https://www.varsitytutors.com/high_school_physics-help/understanding-the-zeroth-law-of-thermodynamics
https://physicsteacher.in/2017/10/01/zeroth-law-thermodynamics-thermal-equilibrium/
https://www.google.com/search?q=zeroth+law+of+thermodynamics+problems+and+solutions&sxsrf=ACYBGNRBSkPdy-C6dLBaH77mMJK8BetJCQ:1573993726098&ei=_jzRXcO3BdaQr7wP-7KmiAs&start=10&sa=N&ved=2ahUKEwjDrrDHn_HlAhVWyIsBHXuZCbEQ8tMDegQIDRAw&biw=1707&bih=821
https://braingenie.ck12.org/skills/105642
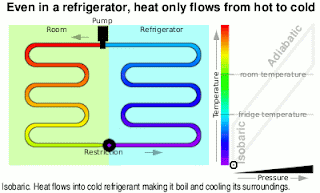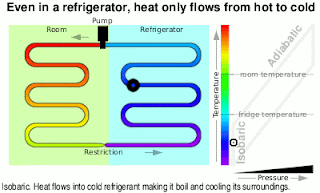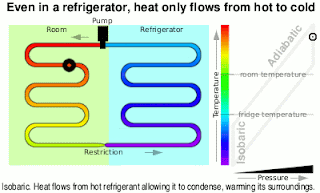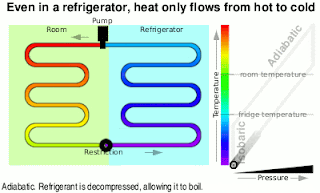







Wonderful content!
ReplyDeleteI rate this blog 10/10
John Paul L. Catchuela
Great job for the detailed material !
ReplyDeletei'll rate this 10/10
A very detailed study material.
Deletevery helpful keep it up
gonna give it a rate of 10/10
Alicio Lapasaran Jr.
BSEE/TIP
Thanks it helped me in studying for my exam tomorrow! Thumbs up a 10/10
ReplyDelete10/10! sana may ganito na noon pa para mas madali kong naunawaan itongntopic na ito.
ReplyDelete10/10!
Ayos! Mas naintindihan ko zeroth law because of this blog. Up for this! 10/10
ReplyDeleteOwen Tabuga
BSECE/TUP-Taguig
Informative and very helpful. It tackles the theory and concepts very well and also provides example to clearly understand the topic.
ReplyDeleteI'll rate this blog 11/10
Den Carl De La Cruz
BSECE/TUP-Taguig
Simple yet effective.
ReplyDelete9/10
Adonis Ramos
BSECE-TUPT
As usual great a blog. The blogger seeks to simplify the explanation of the formulas used in second law of thermodynamics without using calculus which is great. 10/10
ReplyDeleteNice. I gained a lot of knowledge in this blog. Cool. 10/10
ReplyDeleteVery useful! Hoping for more of this
ReplyDeleteUseful and informative. Keep updating your blogspot. Godbless
ReplyDeleteAngelica Mendoza
BSCE-3
useful blog :)
ReplyDeleteThe information was helpful and easy to understand for those students and seeks answers.
ReplyDeleteKristi Errl Mari Cortez
3rd Yr/ BS ECE/ TUP-T
Informative and easy to understand, veeyry helpful to students taking this course.
ReplyDelete10/10
4se bsee
Now I have understood the topic well because of the great content of this blog. Thank you!
ReplyDeleteHj Apino
2nd yr/BGT-AT/TUP-T
I understood more from than this since I was absent. Amazing!
ReplyDelete10/10
Altaire Reyes
Freshman
BS CpE 191
Very educational, the usage of graphic illustrations and video presentation provides better interpretation of the subject. 10/10.
ReplyDeleteVery informative very nice work
ReplyDeleteWell done
ReplyDeleteWell done!
ReplyDelete10/10