FIRST LAW OF THERMODYNAMICS
Law Conservation Of Energy
2. In electric ovens, the electric energy is converted into heat energy.
We know that energy exists in different forms such as nuclear energy,chemical energy, electric potential energy, elastic energy, radiant energy, thermal energy and sound energy etc. All these energies can be changed from one form to another form. However, these energies cannot be created or destroyed.
NOTE:
- Energy can neither be created nor destroyed.
- It only changes from one form to another.
- the total amount of energy remains constant in a system.
EXAMPLE OF LAW CONSERVATION OF ENERGY.
1. When the driver applies the brakes on a moving car, the brakes will be rubbed against the car wheels. Hence the mechanical energy of a car is changed in to heat energy due to friction between the brakes and moving car wheels.
3. During charging of battery, the electrical energy is converted into chemical energy.
Some ideas coming from youtube to help us learn more about Law Conservation of Energy
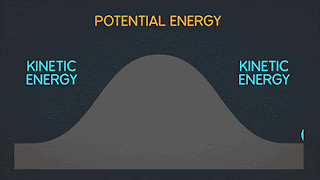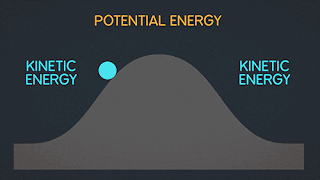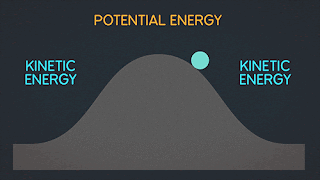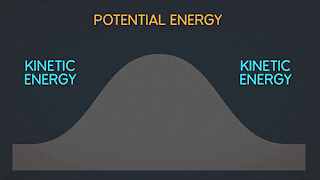
WHAT IS POTENTIAL ENERGY ?
Potential energy is the latent energy in an object at rest, and is one of two forms of energy. The other form, kinetic energy, is the energy expressed by an object in motion. Potential energy is a core concept of any physics-based discussion, and one of the most influential variables in the formulas that describe our known universe.
Potential energy is essentially what it sounds like, though there are a few intricacies involved. The actual potential energy of an object depends on its position relative to other objects. For example, a brick has more potential energy suspended off of a two-story building than it does resting on the ground. That's because the brick's relative position to the Earth gives it more energy. Two bricks next to each other don't give each other more energy though, because there is no force acting on them.
The same principle can be applied on any scale, be it galactic or atomic. Indeed, atoms possess potential energy as well, though their constant movement shifts much of their potential energy into kinetic energy.
NOTE:
The formula for potential energy depends on the force acting on the two objects. For the gravitational force the formula is P.E.=mgh, where m is the mass in kilograms, g is the acceleration due to gravity (9.8 m / s2 at the surface of the earth) and h is the height in meters. Notice that gravitational potential energy has the same units as kinetic energy, kg m2 / s2. In fact, all energy has the same units, kg m2 / s2, and is measured using the unit Joule (J).
Problem set:1 Potential energy
A box has a mass of 5.8kg. The box is lifted from the garage floor
and placed on a shelf. If the box gains 145J of Potential Energy (Ep),
how high is the shelf?
Solution:
use:Ep = mgh
Ep = potential energy (Joules)
m = mass of box (kg)
g = gravitational field strength (N/kg)
h = difference in height (m)
rearrange equation to find height
h =
Ep/mg
=
145/5.8x10
=
145/58
= 2.5
Answer:The shelf is 2.5m high
Problem set:2 Potential energy
A man climbs on to a wall that is 3.6m high and gains 2268J of
potential energy. What is the mass of the man?
Solution:
Use:
Ep = mgh
Rearrange to get an equation for m.
m=
Ep/gh
=
2268/10x3.6
=
2268/36
= 63
Answer: So the mass of the man is 63kg.
WHAT IS KINETIC ENERGY ?
Kinetic energy is energy possessed by an object in motion. The earth revolving around the sun, you walking down the street, and molecules moving in space all have kinetic energy.
NOTE:
KE = ½ mv2
KE = 140J
m = ?
v = 21m/s
2KE/v2 = m OR m = 2KE/v2 (rearrange equation)
m = 2(140J)/(21)
2
m = 280J/441
Answer:
m = 0.63kg
WHAT IS MECHANICAL ENERGY ?
|
Mechanical energy is due to the position or movement of an object. The formula for mechanical energy is mechanical energy = kinetic energy + potential energy.
Objects that have mechanical energy
Every moving object in the universe has the mechanical energy. For example a
train moving on the track has mechanical energy. This is due to its motion
.
A book placed on a table above the ground at height ‘h’ has mechanical energy
A book placed on a table above the ground at height ‘h’ has mechanical energy
(potential energy). This is due to its position above the ground.
Short Quiz
1. What is Law of Conservation of Energy?
Answer :
Energy can neither be created nor destroyed. It only changes from one form to another.
2. What is Kinetic energy?
Answer :
energy possessed by an object in motion.
3.What is Potential energy?
Answer :
the latent energy in an object at rest, and is one of two forms of energy
4.A 0.5 kg ball is about to roll off the edge of a 1.5m tall table what it is current potential energy ?
Answer :
7.35 J
5.What is the Kinetic Energy of a 100 kg object that is moving with a speed of 12.5 m/s?
Answer :
KE = 7.8x10^3
6. A skier is at the top of a hill. at the bottom of the hill she has a velocity of 12m/s how tall was the hill ?
Answer:
7.35 m
7. A 3000 J heat is added to a system and 2500 J of work is done by the system. what is the change in internal energy of the system ?
Answer: internal energy increases by 500 J
8. what is the change in internal energy of a car if you put 12.0 gal of gasoline into its tank the energy content of the gasoline is 1.310^8 J/gal All other factors such as cars temperature are constant ?
Answer :
1.610^9 J
9. The internal energy of a system decreases by 200J. If 50 J or work in done BY the gas, how much energy is transfered as heat? Is the work done positive or negative?
Answer :
-150J; work done is positive.)
10.A gas in a closed container is heated with 10 J of energy causing the lead of the container to rise 2m with 3N of force what is the total change in energy of the system ?
Answer : 4 J
References:
https://msbclasses.weebly.com/uploads/1/0/0/4/100402086/kinetic_energy_practice_questions_answer_key.pdfhttps://www.physicsclassroom.com/class/energy/Lesson-1/Mechanical-Energy






A great learning material!
ReplyDeletei'll rate this blog 10/10
-Joshua Vida
Great explanations!
ReplyDeleteI rate this blog 10/10
John Paul L. Catchuela
Thank you for sharing this information. It helps a lot.
ReplyDeleteAn informative blog.. thanks mate 10/10
ReplyDeleteThis is a very informative blog.
ReplyDeletewith precise definition example and formulas that helps students or readers to understand further the concept. Good job.
I'll rate this blog 10/10
Alicio M. Lapasaran Jr.
BSEE/TIP
nice and precise! mas naintindihan ko na sya
ReplyDelete10/10
Hubert Desacula
BSECE/TUP
Brief explanations but very informative. I'll rate this blog 10/10
ReplyDeleteOwen Tabuga
BSECE/TUP-Taguig
Informative and very helpful. It tackles the theory and concepts very well and also provides example to clearly understand the topic.
ReplyDeleteI'll rate this blog 11/10
Den Carl De La Cruz
BSECE/TUP-Taguig
Simple yet effective.
ReplyDelete9/10
Adonis Ramos
BSECE-TUPT
Very simple and informative blog. Use of practical examples helps reader understand the First Law of Thermodynamics. 10/10
ReplyDeleteJohn Dino Pangilinan
BSEE TUPT
10/10 Another one. Very informative.
ReplyDeleteAngelica Mendoza
BSCE-3
The sample problem are great tool to apply all the principles tackled in you presentation. This will also help them to familiarize the equation presented
ReplyDeleteexamples helps the reader to understand more 10/10
ReplyDeleteVery informative and well explained.
ReplyDeleteKristi Errl Mari Cortez
3rd Yr/ BS ECE/ TUP-T
Informative and easy to understand, veeyry helpful to students taking this course.
ReplyDelete10/10
4se bsee
It is hard to understand this topic especially in my physics class but due to the great visuals and informative contenrt, I have understood more about this topic.
ReplyDeleteHj Apino
2nd yr/BGT-AT/TUP-T
Well explained! 10/10
ReplyDeleteThis content of this blog is well explained in terms of example, illustrations, videos and ideas. Good job. 10/10
ReplyDelete